Study: Screening for pancreatic cancer detects early-stage disease and improves survival
A research study has shown that screening for pancreatic cancer in people with an inherited mutation or family history was able to detect early-stage pancreatic cancers and improve survival. These results will likely change pancreatic cancer screening guidelines for high-risk individuals (Posted 8/30/22)
Este artículo está disponible en español.
Contents
| At a glance | Questions for your doctor |
| Study findings | Guidelines |
| Strengths and limitations | Clinical trials |
| What does this mean for me? | Related resources |
STUDY AT A GLANCE
What is this study about?
The Cancer of the Pancreas Screening (CAPS) program, which began in 1998, is an ongoing study looking at whether screening for pancreatic cancer in high-risk individuals (those with an and/or family history) can lead to the diagnosis of pancreatic cancer when it is most treatable. This study reported on the outcomes for the most recent group of participants in the study, which continues to enroll patients. This study also updated survival outcomes for previous CAPS participants.
Why is this study important?
Experts predict that pancreatic cancer will be the second most common cause of cancer death in the United States by 2026. The 5-year survival rate for people diagnosed with pancreatic cancer is low, due in part to diagnoses.
Pancreatic ductal is the most common type of pancreatic cancer. It is seldom found at a treatable because people usually experience symptoms only with advanced disease. Few studies have been conducted to understand how pancreatic cancer screening affects diagnosis and survival outcomes for high-risk individuals; the limited studies that have been done produced promising results.
This study confirms the findings of previous studies that show screening can lead to diagnosis of early- pancreatic cancer and increased survival. These results will likely change pancreatic cancer screening guidelines for high-risk people. The American Society for Gastrointestinal Endoscopy released a new guideline in early 2022 that recommends pancreatic cancer screening for high-risk people because screening was associated with earlier detection, better survival outcomes and fewer adverse events.
Pancreatic cancer screening in CAPS study
The CAPS study enrolled 1,461 people who were at high risk for pancreatic cancer. High-risk participants included individuals with inherited mutations in , , , , , CDNK2A, a gene associated with (, , , or ). It is important to note that other than participants with inherited mutations in and , all gene carriers also had a family history of pancreatic cancer in at least one (parent, sibling or child) and one-second degree relative (aunt, uncle, grandparent, grandchild, niece or nephew).
Participants in the CAPS studies had yearly imaging of their pancreas with either an endoscopic (EUS) or MRI/MRCP. EUS involves passing an endoscope, a tube-like instrument with a light and a lens for viewing and an attached probe down the esophagus to the stomach. This allows doctors to look closely at the pancreas (see image below). EUS is performed as an outpatient procedure under light anesthesia.
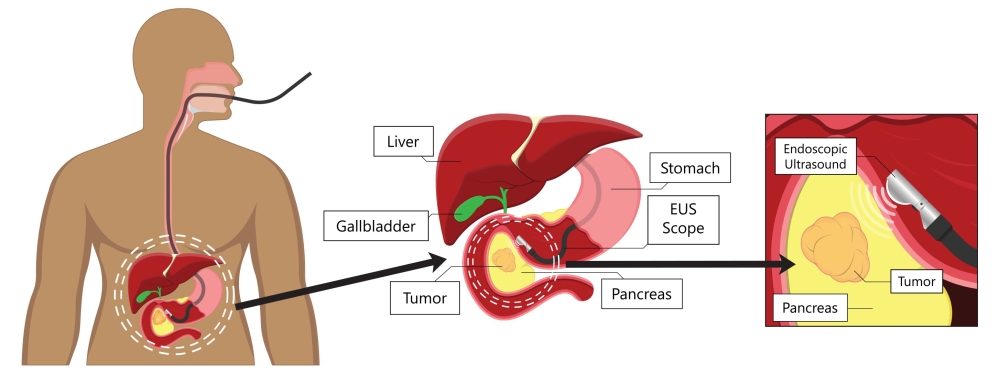
Participants also had a type of imaging known as MRCP (magnetic resonance cholangiopancreatography) every two years. An MRCP is a special type of that helps visualize the liver, gall bladder, pancreas and their ducts.
Although not used in this study, another type of screening, endoscopic retrograde cholangiopancreatography, or ERCP, can also be used. During an ECRP, an endoscope, is passed through the mouth and down into the first part of the small intestine. A smaller tube (catheter) is then inserted through the endoscope into the bile and pancreatic ducts. A dye is injected through the catheter into the ducts, and images are taken.
Each year, participants also donated a blood sample that the researchers are using to help develop new blood-based tests for early detection of pancreatic cancer.
Study findings
During the study, nine of the 1,461 participants were diagnosed with pancreatic cancer that was detected by screening. Seven of the nine people diagnosed (78 percent) had I pancreatic cancer. Experts estimate that without screening, only about 5 percent of pancreatic cancers are diagnosed at I. This finding suggests that screening high-risk people may significantly improve early detection of the disease.
Of the nine participants diagnosed with pancreatic cancer:
- Five had a family history of pancreatic cancer but had no known , highlighting the importance of considering family history, rather than just genetic test results when making decisions about high-risk screening for pancreatic cancer.
- Three had an and a family history of pancreatic cancer.
- One had an with no family history.
To date, among all five CAPS (1-5) studies (including this one), 26 of 1,731 high-risk participants have been diagnosed with pancreatic cancer. The cancers found in 19 of these 26 participants were detected through screening. More than half (58 percent) of the participants whose cancers were detected during screening had I disease.
Participants with screening-detected cancer also had longer survival outcomes than those whose cancer was due to concerning symptoms rather than screening. Patients diagnosed with pancreatic cancer during screening survived an average of almost 10 years, while people diagnosed outside of screening survived an average of 1.5 years.
Strengths and limitations
Strengths
- This is a large, phase 3 clinical trial being conducted at multiple centers.
- This study has been ongoing for more than 20 years, giving researchers time to detect more cancers if they were going to occur. A shorter study might have had less informative results.
Limitations
- The follow-up period for the CAPS-5 group was relatively short—on average four years—so it is difficult to draw conclusions about long-term survival for this group. Longer follow-up time is needed to fully understand the benefits of endoscopic and MRI-based screening in high-risk individuals.
- Only 26 pancreatic cancers have been diagnosed during the entire CAPS program. This small sample size limits the reliability of the results. As more people are diagnosed with pancreatic cancer by screening and have long-term follow-up, patterns in at diagnosis and survival rates will become clearer.
- The participants were mostly white (94.5%) so the results might not be generalizable across diverse populations.
What does this mean for me?
If you have a family history of pancreatic cancer or an that increases your risk, you may benefit from pancreatic cancer screening. Annual screening with and/or endoscopic may detect precancerous or disease when it is most treatable.
If you do not know if you have an , genetic testing might help you decide if screening for pancreatic cancer is right for you. Additional information can be found on the FORCE page for pancreatic cancer screening guidelines.
Deciding at what age to begin screening depends on your family history and which you carry. Ask your doctor or a genetic counselor about your risk of pancreatic cancer and other cancers, given your personal and family history.
References
Dbouk M, Katona BW, Brand RE, et al., The multicenter Cancer of Pancreas Screening study: impact on and survival. Journal of Clinical Oncology; 2022; JCO-22. Published online June 15, 2022.
Johns Hopkins Medicine Department of Pathology. Cancer of the Pancreas Screening Study. Accessed August 1, 2022.
Sawhney MS, Calderwood AH, Thosani NC, et al., ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: summary and recommendations. Gastrointestinal Endoscopy; 2022; 95, no. 5: 817–826. Published online February 16, 2022. DOI: 10.1016/j.gie.2021.12.001.
Disclosure: FORCE receives funding from industry sponsors, including companies that manufacture cancer drugs, tests and devices. All XRAYS articles are written independently of any sponsor and are reviewed by members of our Scientific Advisory Board prior to publication to assure scientific integrity.
Share your thoughts on this XRAY review by taking our brief survey.
posted 8/30/22
This review was updated on 09/15/2022 to correct typo describing changes in eligibility.
IN-DEPTH REVIEW OF RESEARCH
Study background
Some individuals have an increased risk for pancreatic cancer because of an or a family history of the disease. Pancreatic cancer is rare in the general population, and people are considered to be at high risk if they have a five percent or higher lifetime risk. Currently, no effective screening methods exist for detecting pancreatic cancer. As a result, pancreatic cancer is one of the deadliest cancers worldwide.
Johns Hopkins University began its Cancer of the Pancreas Screening (CAPS) studies in 1998 to determine “the effectiveness of early detection screening in high-risk individuals of pancreatic cancer" and to discover "new biomarkers to improve early detection.” Over the past two decades, the CAPS study and others have shown that screening for pancreatic cancer in high-risk individuals without symptoms has the potential to detect the disease in its early stages and improve survival outcomes. The most recent CAPS study (CAPS5) confirms and strengthens these findings.
Researchers of this study wanted to know
The researchers of this study wanted to evaluate the outcomes (cancer diagnosis and survival) among high-risk patients undergoing surveillance for pancreatic cancer.
Populations looked at in this study
Participants in the CAPS-5 study were known to have either an that increased their risk for pancreatic cancer (44 percent: 643 of 1,461 participants) or a strong family history of the disease but no know mutation (56 percent: 818 of 1,461 participants).
- Among participants with inherited mutations:
- 18.4% (269) had a mutation and at least one first- or with a history of pancreatic cancer.
- 4.7% (68) had a mutation with at least one with a history of pancreatic cancer.
- 4.7% (69) had a mutation, which is associated with Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM). People with FAMMM have an increased lifetime risk of developing melanoma and pancreatic cancer.
- 4.0% (58) had with at least one first- or with a history of pancreatic cancer.
- 4.2% (62) had a mutation with at least one first- or with a history of pancreatic cancer.
- 6.4% (93) had an mutation with at least one first- or with a history of pancreatic cancer.
- 1.2% (18) had an mutation, which is associated with .
- 0.4% (6) had more than one and at least one first- or with a history of pancreatic cancer.
- Among participants who did not have a known but had a family history of pancreatic cancer:
- 23.7% (346) had at least two or more first-degree relatives with a history of pancreatic cancer.
- 27.5% (402) had one and one or more second-degree relatives with a history of pancreatic cancer.
- 0.3% (5) had one with young-onset pancreatic cancer (50 years old or younger).
- 4.5% (65) met other criteria which allowed them to be included in the high-risk group.
- Participant demographics:
- The average age of participants was 60.3 years.
- 31.1% had a personal history of cancer (mostly breast cancer).
- 32% were never/former smokers.
- 3.9% were current smokers.
- 51.8% used alcohol.
- 9.8% had a history of diabetes (type 1 or 2).
- 64.6% were female.
- 94.5% were white.
- 3.5% were Black.
- 1.3% were Asian.
- 2.4% were Hispanic/Latino.
- 0.7% identified as other/multiple.
Study design
The CAPS-5 study is a study with participants from eight medical centers in the United States. Enrollment opened in 2014 for individuals that met the high-risk and age criteria. The authors of this study report on all participants enrolled through June 2021.
Screening consisted of yearly imaging of the pancreas with either an endoscopic (EUS) or magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP). Each year, participants also donated a blood sample that was used for research purposes. If the medical team found significant lesions, participants had more frequent surveillance (every 3 to 6 months) to monitor changes. If the team suspected cancer or precancer, participants had surgery and treatment based on current recommendations.
The study also included a group of people with pancreatic disease and a group of healthy people to compare to participants in the high-risk group. Patients with pancreatic disease had pancreatic cancer, pancreatic cysts or chronic pancreatitis (inflammation of the pancreas). Healthy people were patients with a normal pancreas who were undergoing routine upper GI endoscopy (EGD), screening colonoscopy or endoscopic retrograde cholangiopancreatography (ERCP) as part of their standard medical care.
Study findings
CAPS-5 study
Nine participants were diagnosed with pancreatic cancer detected by screening during the study:
- 7 had I cancer:
- 4 of the 7 had a family history of pancreatic cancer with no known .
- 2 of the 7 had inherited mutations and a family history of pancreatic cancer (, ).
- 1 of the 7 had inherited mutations with no family history of pancreatic cancer ().
- 1 had IIB cancer:
- This person had a family history of pancreatic cancer with no known .
- 1 had III cancer:
- This person had an and a family history of pancreatic cancer ().
- 8 had surgically resectable cancer.
- 7 of the 9 participants who were diagnosed with pancreatic cancer are still alive with a median survival of 3.84 years at the time this study was written.
Eight participants had surgery for concerning cystic lesions on the pancreas:
- 3 had high-grade dysplasia (precancer that is likely to become cancerous).
- 5 had low-grade dysplasia (precancer that is unlikely to become cancerous).
One participant discontinued surveillance, then developed symptoms and was diagnosed with IV pancreatic cancer four years later.
Combined CAPS1-5 studies
Twenty-six participants have been diagnosed with pancreatic cancer since the program began in 1998.
- 38.5% had a known .
- 19 had cancers that were detected by screening, of which:
- 57.9% were I.
- 15.8% II.
- 21.1% III.
- 5.2% IV.
- 7 had cancers that were detected outside of surveillance, of which 85.7% were IV.
- Participants with screening-detected pancreatic cancer had an overall 5-year survival of 73.3% (compared to 5 to 10% in participants with cancer detected outside of surveillance).
Strengths and limitations
Strengths
- This is a phase 3 clinical trial. It is a multicenter, interdisciplinary study that has been ongoing for more than 20 years.
- The study includes individuals with a mix of different mutations and family histories associated with an increased risk of pancreatic cancer.
Limitations
- The follow-up period for the CAPS-5 cohort was short ( median 4 years) so it is difficult to draw conclusions about long-term survival for this group. Longer follow-up time is needed to fully understand the benefits of endoscopic and MRI-based surveillance in high-risk individuals and to evaluate the usefulness of blood tests in early disease detection.
- A relatively small number of pancreatic cancers (26) have been diagnosed during the entire CAPS program. This small sample size limits the reliability of the results. As more people are diagnosed with pancreatic cancer by screening and followed up long-term, patterns in at diagnosis and survival rates will become clearer.
- The participants were mostly white (94.5%) so the results might not be generalizable across diverse populations.
Context
In recent years, screening for pancreatic cancer has shown promise in detecting the disease early, when it is most treatable. However, that conclusion is based on a small number of studies and a small number of pancreatic cancer cases because pancreatic cancer is relatively rare even in high-risk populations. Additionally, most of these studies do not yet have long-term follow-up results, making it hard to predict how screening affects long-term survival.
Current evidence from the CAPS studies and others suggests that screening can be beneficial for high-risk individuals, and the results of the most recent study will likely change screening guidelines for this population. The American Society for Gastrointestinal Endoscopy (ASGE) released a new guideline in early 2022 that recommends pancreatic cancer screening for people with an increased risk for developing the disease. ASGE based their guideline on studies in this field that showed screening for pancreatic cancer in high-risk groups was associated with earlier diagnosis, better survival outcomes and few adverse events.
Conclusions
This study confirms that screening for pancreatic cancer in asymptomatic, high-risk individuals can lead to earlier detection of the disease and better survival outcomes. The results may change national screening guidelines for high-risk populations from “consider screening” to “recommend screening.”
Share your thoughts on this XRAY review by taking our brief survey.
posted 8/30/22
This review was updated on 09/15/2022 to correct typo describing changes in eligibility.
National Comprehensive Cancer Network (NCCN) Guidelines
The NCCN recommends the following for people at increased risk for pancreatic cancer:
- Discuss the benefits and risks of pancreatic cancer screening with their doctor. Screening should be performed by a facility that is experienced with pancreatic cancer screening. The recommended age for considering screening depends on a person’s family history of pancreatic cancer and varies by type of gene mutation.
- Consider screening with magnetic resonance cholangiopancreatography (MRCP) and/or endoscopic (EUS).
- Consider participating in a pancreatic cancer screening study.
The NCCN recommends that people with inherited mutations in the following genes (with or without a family history of cancer) "consider pancreatic cancer screening" with MRCP or EUS:
- (): Consider pancreatic cancer screening every 1-2 years, beginning at ages 30-35 or 10 years younger than the earliest pancreatic cancer in the family.
- : Consider pancreatic cancer screening beginning at age 40 or 10 years earlier than the earliest pancreatic cancer diagnosis in the family.
- and : Consider pancreatic cancer screening beginning at age 50 or 10 years earlier than the youngest case of pancreatic cancer in the family.
NCCN guidelines recommend that people with an in , , , , , , or and a family history of cancer "consider pancreatic cancer screening" with MRCP or EUS, beginning at age 50 or 10 years earlier than the earliest pancreatic cancer diagnosis in the family.
The NCCN does not currently recommend pancreatic cancer screening for people with the above mutations who do not have a family history of cancer.
American Society for Gastrointestinal Endoscopy (ASGE) Guidelines
In February 2022, the ASGE released updated guidelines on pancreatic cancer screening for people with a or mutation. These guidelines recommended:
- All patients with a mutation, regardless of a family history of pancreatic cancer, should undergo annual screening for pancreatic cancer with MRI/MRCP or EUS, beginning at age 50 or 10 years earlier than the earliest pancreatic cancer in the family.
Updated: 10/23/2024
- Based on my or family history, do I have an increased risk of pancreatic cancer?
- Where can I find a clinic that has experience with screening for pancreatic cancer?
- At what age should I begin screening for pancreatic cancer?
- What are the risks and benefits of screening for pancreatic cancer?
- Will my health plan cover the costs of pancreatic cancer screening?
- Do I qualify for any pancreatic cancer screening clinical trials?
The following studies are looking at risk management for pancreatic cancer:
- NCT04970056: Pancreatic Cancer Early Detection for People at High Risk (PRECEDE). The study will collect clinical information, family history and samples (blood, saliva or cheek swab) from people and families at risk for pancreatic cancer.
- NCT02000089: Pancreatic Cancer Screening Study (CAPS5). The CAPS5 study is looking at screening for early cancer in people with an elevated lifetime risk of developing pancreatic cancer who are undergoing screening with endoscopic , MRCP or .
- NCT03250078: A Pancreatic Cancer Screening Study in Hereditary High-Risk Individuals. The main goal of this study is to screen and detect pancreatic cancer and precursor lesions in individuals with a strong family history or genetic predisposition to pancreatic cancer. Magnetic Resonance Imaging and Magnetic Cholangiopancreatography (MRI/MRCP) will be utilized to screen for pancreatic cancer or precursor lesions.
- NCT03568630: Blood Markers of Early Pancreas Cancer. This study looks at whether identifying biomarkers of early pancreatic ductal () could facilitate screening for individuals with higher-than-average risk, expedite the diagnosis in individuals with symptoms and substantially improve an individual's chance of surviving the disease.
- NCT03250078: A Pancreatic Cancer Screening Study in Hereditary High-Risk Individuals. The goal of this study is to use Magnetic Resonance Imaging and Magnetic Cholangiopancreatography (MRI/MRCP) to screen for pancreatic cancer in individuals with a strong family history or genetic risk .
Other clinical trials for pancreatic cancer screening and prevention may be found here.
Updated: 12/23/2024
The following organizations offer peer support services for people with or at high risk for pancreatic cancer:
- FORCE peer support
- Our Message Boards allow people to connect with others who share their situation. Once registered, you can post on the Diagnosed With Cancer board to connect with other people who have been diagnosed.
- Peer Navigation Program will match you with a volunteer who shares your mutation and situation.
- Private Facebook Group
- Virtual and in-person support meetings
- Join a Zoom community group meeting.
- LGBTQIA
- Men
- American Sign Language
- People of Color
- Spanish-speakers
- PanCAN
- Let's Win PC
- The Healing NET Foundation is a nonprofit organization for people with neuroendocrine cancers.
- The Neuroendocrine Cancer Awareness Network (NCAN) is a non-profit organization dedicated to raising awareness of neuroendocrine cancer and providing support for caregivers and people with NETs.
Updated: 08/23/2022
- The Collaborative Group o the Americas on Inherited Gastrointestinal Cancer (CGA-IGC) curates an updated list of hospitals and programs with expertise in pancreatic cancer screening for high-risk people.
- The PROCEDE Consortium is a collaboration of experts working to improve the detection and prevention of hereditary pancreatic cancer.
Register for the FORCE Message Boards to get referrals from other members. Once you register, you can post on the Find a Specialist board to connect with others who share your situation.
Updated: 10/23/2024
Who covered this study?
U.S. News & World Report
Regular screening pays off for people at high risk for pancreatic cancer 



 This article rates 5.0 out of
5 stars
This article rates 5.0 out of
5 stars
The Science Times
Early Detection of Pancreatic Cancer Through Regular Screening Increases the Patient's Chance to Live Longer 



 This article rates 5.0 out of
5 stars
This article rates 5.0 out of
5 stars
Johns Hopkins Medical Newsroom
Regular screening of people at high risk for pancreatic cancer pays off 



 This article rates 4.0 out of
5 stars
This article rates 4.0 out of
5 stars


